Proteins are essential for life - from immune signalling to digestion, almost every biological process relies on them. But proteins can be fragile. Heat, stress, and chemical environments often cause them to unfold and stop functioning. For industrial biotechnology and medicine, this instability is a major bottleneck. Creating proteins that remain stable under harsh conditions can increase efficiency, reduce production costs, and improve therapeutic durability.
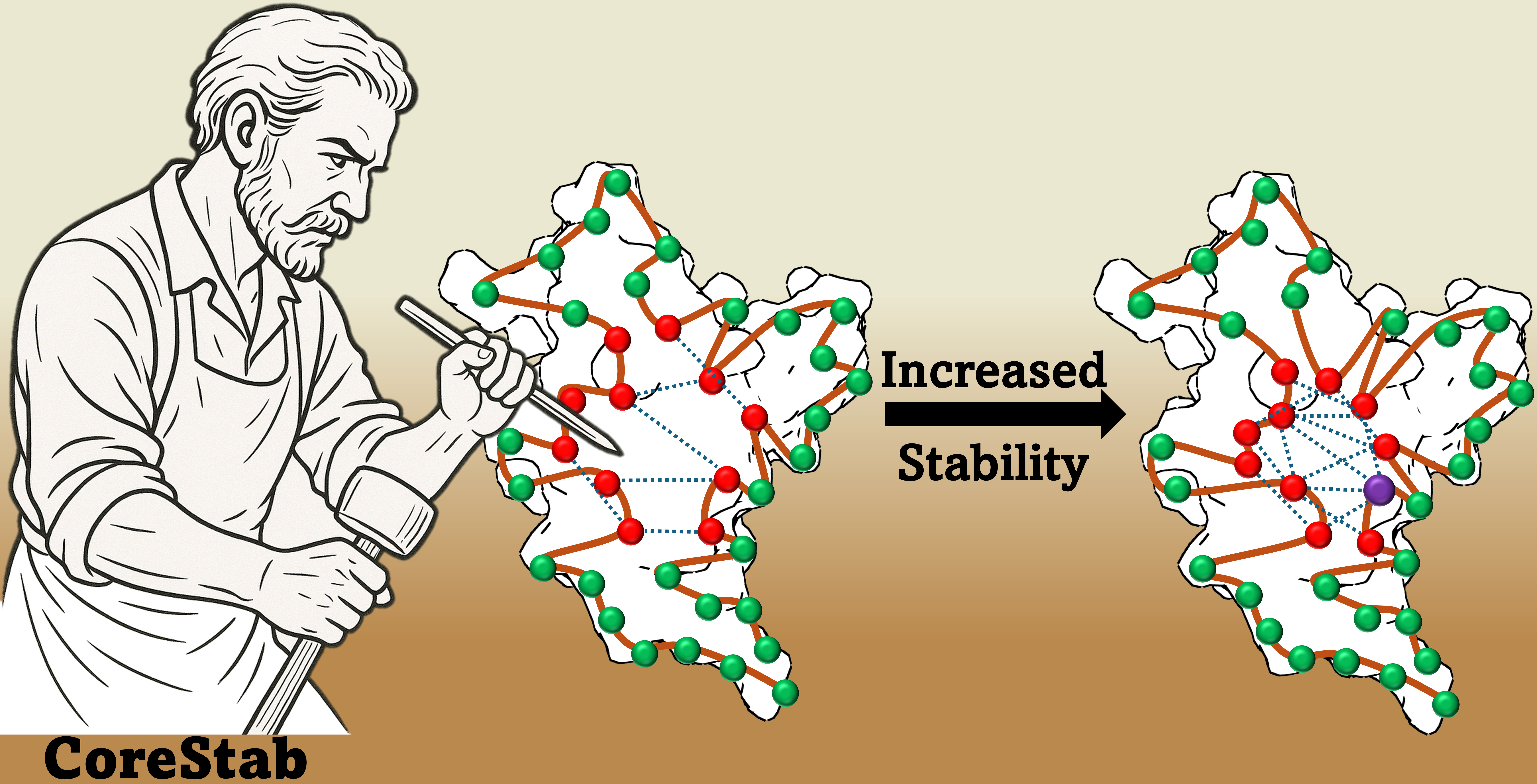
A recent study from NCBS sought a way to strengthen proteins without disrupting their function. The research team focused on the hydrophobic core of proteins. This core is made of water-hating amino acids that huddle together tightly to avoid water. This packing helps a protein keep its shape. But sometimes, the packing is not perfect as tiny empty gaps can form inside during folding. These small gaps make proteins weak, like building a wall with small holes - it can crumble easily. The research team wondered: What if we could fill these gaps with slightly bigger pieces? Would the protein become more stable?
“We built a computer program called CoreStab. It looks at the 3D shape of a protein and suggests small changes where a slightly bigger amino acid can fit better in the core,” says Dr Aravind Ravichandran, the lead author of the study. The program is careful not to perturb the protein’s function.
To test this, the team used a protein called NEDD8. It looks almost the same as another protein named ubiquitin, but NEDD8 is much less stable. CoreStab found two places inside NEDD8’s core where the amino acid valine was a bit too small, leaving extra space around it. CoreStab suggested replacing valine with a slightly larger amino acid called isoleucine. When the scientists performed the replacement, protein stability improved.
“The protein’s melting temperature increased by 17°C. It became much tougher without losing its normal function,” says Aravind.
How does filling a tiny gap hold the whole protein together? Isoleucine’s skeleton is one carbon atom longer than valine’s. The research team showed that this extra carbon atom filled the gap and improved the core’s packing, lowered flexibility, reduced internal tensions, and strengthened long-range interactions across the protein. In other words, the tiny interior upgrades ripple outward, making the entire structure more robust.
“This shows that improving a protein doesn’t always mean big changes. Small, well-planned edits inside the protein can make it survive heat and stress much better,” says Dr Ranabir Das, the principal investigator of the study. “With CoreStab, scientists can now rapidly test stability-enhancing mutations on many proteins, potentially designing stronger enzymes for industry and more durable protein therapeutics for healthcare. Future studies will explore combining such subtle internal edits with other engineering strategies to tailor proteins for tougher environments,” he added.





0 Comments