Researchers from the National Centre for Biological Sciences (NCBS), Bangalore, have developed a mouse model for detecting cells that respond to antipsychotic drugs in live brain tissues. Using this, the team have also discovered that the antipsychotics Clozapine and Olanzapine affect ependymal cells—a class of brain cells responsible for producing cerebrospinal fluid—previously unknown to be affected by antipsychotics. This unexpected discovery highlights how little we know about the mechanisms by which antipsychotics exert either beneficial or unwanted effects, and the need for more work in this field.
In the early 1950s, a huge advance occurred in the field of psychiatry—the discovery of antipsychotics. The first antipsychotic to be discovered was RP4560, a potential antimalarial compound that was eventually named Chlorpromazine. Chlorpromazine was found to induce a state of calmness without causing sleepiness, or “sedation without narcosis” as described by the young French army surgeon, Henri Laborit, who first tested it on human patients. Although psychiatrists were initially reluctant to test ‘Laborit’s drug’, as chlorpromazine was then known, on patients, this compound eventually helped millions of people worldwide manage mental diseases, and still remains a benchmark drug for treating schizophrenia.
Since the discovery of Chlorpromazine, a host of new antipsychotic drugs have been developed, and much is known about the proteins and receptors that these compounds bind to. However, the mechanisms by which the drugs exert their effects on mammalian brains as yet remain unclear.
Now, Radhika Joshi and Mitradas Panicker from the National Centre for Biological Sciences (NCBS), Bangalore, have developed a system using transgenic mice to identify cells in live brain tissues that respond to antipsychotic drugs. With this system, the team have also identified that Clozapine and Olanzapine (mainly used to treat schizophrenia) affect ependymal cells in the brain. Ependymal cells are a class of brain cells that produce cerebrospinal fluid, and have never before been considered as targets of antipsychotic drugs.
The study, which began as a quest to explore how antipsychotics exert therapeutic and unwanted side effects on the brain, now allows researchers to identify live cells within specific areas of the brain responding to specific types of antipsychotics. By identifying the cells and tracing circuits that change in response to antipsychotics, scientists may soon be able to understand how these drugs affect individual behaviors.
Joshi and Panicker’s work relies on transgenic mice called ‘FosTRAP’ mice, which carry a genetically encoded tool named TRAP (Targeted Recombination in Active Populations), first developed in Professor Liqun Luo’s laboratory at Stanford University. The tool embedded in the mouse’s genome uses a fluorescent protein to permanently ‘light up’ cells responding to a specific stimulus. Using these mice, the team were able to identify groups of brain cells that responded to four drugs – the first generation or typical antipsychotics, Haloperidol and Loxapine, and the second generation or atypical antipsychotics, Clozapine and Olanzapine.
“Previously such cells were only identified in fixed tissue, that is, dead tissue. Our work shows that they can be identified in living tissue, which means that one can interrogate the [brain] circuits in many ways,” says Mitradas Panicker. “Furthermore, this approach identifies the cells that are the targets of these drugs, and not just the proteins involved,” he adds.
Although the study found that most of the brain regions responsive to these drugs were similar to those reported in earlier studies, some intriguing results were also observed. The serotonin receptor, 5-HT2A, to which Clozapine binds with high affinity, is thought to be responsible for this drug’s therapeutic effects. However, in this study, areas in the cortical and thalamic regions responsive to Clozapine remained responsive even in mice lacking the 5-HT2A receptors in their brains. This suggests that other receptors may be interacting with Clozapine, which opens up new routes of enquiry on how the drug exerts its effects on the brain. In addition to this, the work has also identified that the ependymal cells, which line the ventricles of the brain and produce cerebrospinal fluid, are also affected by Clozapine.
“The result with the ependymal cells was a complete surprise. These cells have never before been identified as targets for antipsychotic drugs,” says Radhika Joshi. “But it still remains to be seen if this effect of Clozapine on the ependymal cells has therapeutic effects or is responsible for the side effects of this drug. Our study sets the stage for further exploration that will be needed for this,” she adds.
With different combinations of transgenic mice strains, and specific proteins that can activate or inhibit brain cells, the system described in this study can further be used for detailed explorations of cells and circuits responsive to different types of drugs.
“We have only examined the brain in this case, but it should [also] be possible to examine peripheral tissue in the same model and understand the substantial side effects that are associated with these drugs,” says Panicker.
“Overall, our work has provided the proof-of-concept that cells responsive to drugs can be identified in live tissues,” adds Joshi.
The paper can be accessed at: Identifying the In Vivo Cellular Correlates of Antipsychotic Drugs
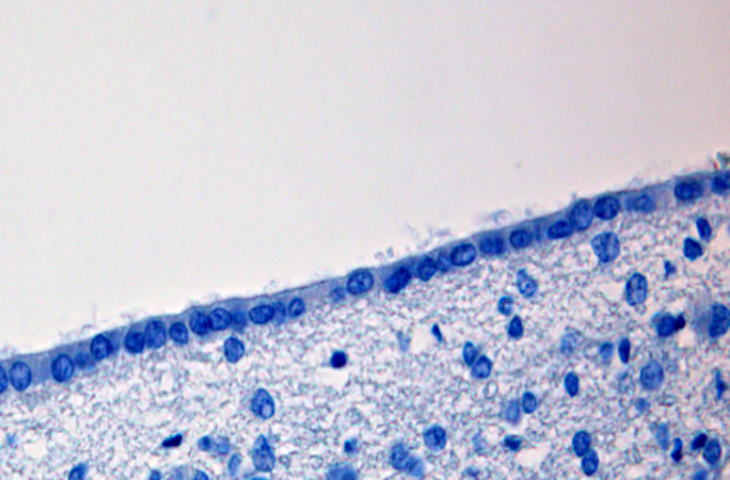
Image: Hematoxylin-stained human ependymal cells seen at 400x magnification. Ependymal cells are a class of brain cells that produce cerebrospinal fluid, and have never before been identified as targets of antipsychotic drugs. However, recent research on mice suggests that the antipsychotic drugs Clozapine and Olanzapine do affect these cells.
Image Credits: Martin Hasselblatt MD (CC-BY-SA-3.0 from Wikimedia Commons)









0 Comments