At least 20 times in the history of evolution, and possibly several times more, single-celled organisms have transitioned into multicellularity, evolving into forms larger than their ancestors. In many such instances, when single-celled organisms went into overdrive, plants, animals and fungi, and other forms came into existence. Multicellular organisms gained significant advantages, such as the ability to move and more efficiently use resources.
However, as these life forms grew larger, they faced a key challenge: how to transport nutrients and oxygen throughout their bodies. In single-celled organisms without swimming or internal active transport mechanisms, such as molecular motors, this transport relies entirely on diffusion, the passive movement of molecules from areas of high concentration to low concentration. But diffusion becomes increasingly inefficient as organisms grow larger, since the surface area-to-volume ratio decreases. Many theoretical models and experiments suggest that diffusion alone can impose a strict size limit on early multicellular life.
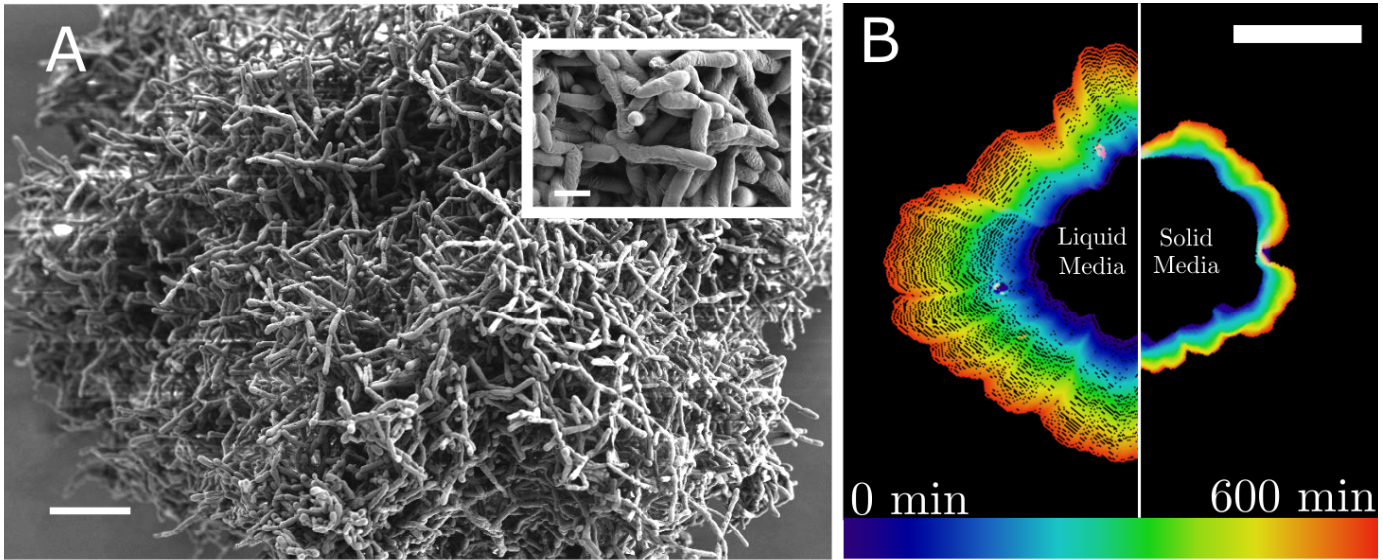
A recent study published in Science Advances from Dr. Shashi Thutupalli's lab at the National Centre for Biological Sciences (NCBS) investigated how early multicellular clusters may have overcome this diffusion barrier to facilitate nutrient transport and exponential growth. The researchers found that even without specialised structures like cilia, clusters of snowflake yeast can generate fluid flows in their environment. These flows, driven by metabolic activity, are fast, comparable to those generated by cilia in more complex organisms, and allow the yeast to sidestep the diffusion limit. This work highlights a potential biophysical mechanism that could have enabled early multicellular clusters to grow larger and more complex before specialised transport systems evolved.
To understand what enables multicellular clusters to grow exponentially beyond the limits of diffusion, the team used snowflake yeast from the Multicellularity Long-Term Evolution Experiment (MuLTEE), running at Georgia Institute of Technology for nearly a decade now, following seminal work by Dr. William Ratcliff in 2012. Over generations, these yeasts evolve into large, visible clusters—millimetres in size—each made up of hundreds of thousands of genetically identical cells.Thutupalli’s team showed that once these clusters reach a certain size, their metabolic activity creates spontaneous density gradients in the surrounding fluid. These gradients generate fluid flows that circulate nutrients deep into the cluster, supporting continued growth even at sizes that should be impossible for diffusion alone.
To investigate how the liquid environment supports such rapid growth, the researchers placed yeast clusters in closed chambers with and without glucose, the yeast’s main metabolic fuel. Without glucose, fermentation halted, and nutrient transport relied solely on diffusion. However, when glucose was present, the yeast produced ethanol and CO₂ as byproducts. Ethanol is less dense than water (789 g/L vs. 1000 g/L), so as it accumulates beneath the cluster, it rises and displaces the water above it. This circulation draws fresh nutrients into the cluster, effectively bypassing the usual diffusion limits.
“At large enough sizes, the amount of ethanol they produce is sufficiently high that it sets up a density contrast with the surrounding environment. Ethanol rises upward, and displaces the water above it. And because the chamber is closed, fluid continuity ensures that the displaced water then circulates back into the cluster”, said the lead author, Nishant Narayanasamy.
This finding suggests that simple physical processes can act as a "biophysical scaffold," enabling multicellular life to grow larger and more complex before the evolution of genetically encoded transport systems.
“Early multicellularity was likely simple, just clusters of cells without differentiation, vasculature, or even cilia. Mechanisms like metabolically driven flows may have helped these primitive organisms grow larger. But as more efficient transport systems like cilia and flagella evolved, they likely outcompeted these simpler strategies, shaping the complex multicellularity we see today”, said Narayansamy. The research highlights that physical and biological processes played a crucial role in driving the evolution of multicellularity and that biophysical processes can influence biological organisation.
“Our study shows that complex and dedicated transport structures/architectures are not necessary for the evolution of large size. It also raises the question of why and how more complex transport mechanisms came up when such simple physical mechanisms are sufficient. Whether the kind of mechanism we have discovered is used by extant organisms and what ecological niches such organisms may occupy are open questions”, said Shashi Thutupalli, senior author of the study.
Link to the study: https://www.science.org/doi/10.1126/sciadv.adr6399









0 Comments