In a recent study, researchers from the National Centre for Biological Sciences (NCBS) have shed new light on the pivotal role of calcium ions (Ca2+) in the functioning of a subset of brain cells that synthesize and release dopamine (dopaminergic neurons) within the brain of fruit flies (Drosophila melanogaster). The study titled "Orai mediated Calcium entry determines activity of central dopaminergic neurons by regulation of gene expression" was conducted by Rishav Mitra and Shlesha Richhariya (former PhD students); in the lab of Prof. Gaiti Hasan, at NCBS, and has been published in the journal eLife. This study investigates a novel molecular mechanism by which influx of calcium ions into a cell is controlled during developmental stages in fruit flies (Drosophila melanogaster). This influx of calcium ions is essential for the maturation of a set of dopaminergic neurons that play a critical role in enabling flight in fruit flies. The findings from this study help understand the principles underlying development and assembly of neural circuitry that supports complex animal behaviors.
Neuronal activity and the transmission of information among neurons rely on the movement of calcium ions. One pathway for calcium entry is by the mechanism of Store Operated Calcium Entry (SOCE). When calcium is released from stores within the cell, the Orai channel on the cell membrane opens, and allows the influx of calcium ions from outside the cell, that are then used to refill cellular calcium stores.
Dopaminergic neurons in the brains of fruit flies play a central role in controlling their ability to fly as adults. These neurons receive signals from other parts of the brain and indirectly also from the environment. They respond by releasing dopamine at an important brain synapse required for sustaining longer bouts of flight.
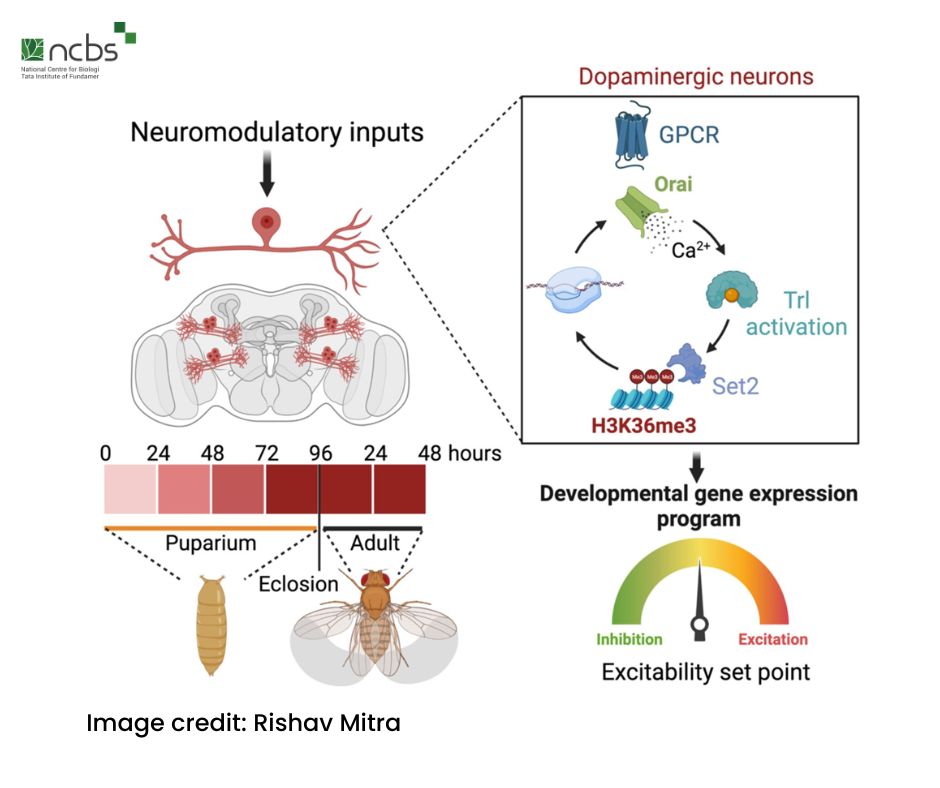
The researchers conducted a series of experiments over the course of the life cycle of fruit flies and identified an interval between the late pupal to early adult stages, as a critical period during which the flight promoting dopaminergic neurons require SOCE. During this period, the presence of calcium ions via SOCE activates a specific set of genes known as SOCE-responsive genes. The expression of these genes is crucial for the maturing nervous system to develop the ability to fly as adults. The hindrance of calcium supply during this stage rendered dopaminergic neurons dysfunctional, as the SOCE-responsive genes remained inactive, resulting in flightless fruit flies.
Further investigation revealed that SOCE affects gene expression by determining the levels of two competing epigenetic signatures on histone 3. The H3K36me3 methyltransferase, a SET domain containing histone modifier, was identified as responsive to SOCE and acted as a transcriptional effector. It enhanced the expression of important GPCRs (receptors for various types of chemicals), other components of calcium signaling and ion exchange channels, ultimately facilitating optimal cellular function required during flight.
The loss of SOCE led to reduced H3K36me3 levels and increased levels of H2K27me3 (another histone modification) which had the opposite effect of gene repression, thus preventing the development of flight in adult fruit flies. However, the researchers discovered that loss of flight could be partially reversed by supplementing the diet of flightless adult flies with a drug that obstructs the action of H2K27me3, temporarily inducing flight.
This finding holds significance, as it could in the future, pave the way for therapeutic treatments for neurodegenerative disorders where imbalances in intracellular calcium signals have been observed such as Parkinson's disease, Huntington's disease, and Spinocerebellar Ataxias.
The researchers also identified a novel SOCE-responsive transcription factor Trithorax-like (Trl) or GAF (GAGA Factor) as playing a pivotal role in allowing dopaminergic neurons to reach optimal excitability during the critical window of time identified earlier. Their research identifies Trl/GAF as the transcription factor activated by SOCE. The SOCE-Trl-Set2 mechanism controls the functioning of various ion exchange channels, ensuring both minimum and maximum levels of excitability, and ultimately, the functional state of dopaminergic neurons in adult fruit flies.
This research not only provides valuable insights on the mechanism of how calcium ions supplied through SOCE influence neuronal function in fruit flies, but also opens up exciting possibilities for future investigations of similar mechanisms in human neurons.
Banner Image: In fruit flies (Drosophila melanogaster), the Store-operated Ca2+ entry (SOCE) channel, Orai, is required for the development of flight-promoting dopaminergic neurons and plays a critical role in downstream gene expression. The study and the above image have been published in the journal eLife.
The published paper can be accessed at the link below
https://elifesciences.org/reviewed-preprints/88808#x-96622600









0 Comments