More than 300 years ago, Leeuwenhoek peered down his handmade lenses, and made mankind’s first-ever observation of bacteria, swimming and squirming around in samples of his own dental plaque. These “animalcules”, as he termed them, appeared to abound in all sorts of samples, ranging across pond water, soil, blood, and tissues. Yet ever since then, scientists have studied bacteria in the lab primarily using either liquid cultures or flat 2D plates. While these methods are convenient and economical, as well as key to the discovery of much of our foundational knowledge on biological processes, they fall short of reflecting several natural environments where bacteria thrive—such as soil, mucus, or within the tissues of plants and animals. As a result, we know very little about how bacterial growth occurs in such complex 3D environments, which are also subject to varying material properties. Understanding this has important real-life consequences for improving agriculture (where bacteria live in soil), treating infections (where pathogenic bacteria invade tissues), and understanding ecological events (since bacterial communities are a critical component of most ecosystems).
A recent study from the National Centre for Biological Sciences (NCBS) has taken a groundbreaking step forward by using 3D models to investigate how bacteria grow and survive in different physical environments. The study's findings reveal that the shape of a bacterium can significantly influence its ability to endure and thrive, highlighting the complexity of survival strategies in a three-dimensional world. This research is the first to show that the physical environment of a bacterium can directly act as a potent selective pressure by favouring certain species over others in a highly predictable manner.
“From decades of past research using simple liquid or flat plates, we have learnt that mutations, chemical signals, and behavioural patterns all affect bacterial physiology. But bacteria inhabit a wide range of environments—from the soil beneath our feet to the mucus lining our guts. So, we wondered: how would such physical differences in their environment impact their survival?” explains Sreepadmanabh M, the study's lead author.
Recognizing that standard lab-grown bacteria might have evolved adaptations for optimal growth in liquid or 2D conditions, the research team focused on a different approach. Working with Deepa Agashe’s lab, they isolated various bacterial species directly from the guts of beetles, allowing them to study these organisms in conditions that more accurately reflect their natural habitats.
To engineer artificial laboratory mimics of the mucus-like mechanical environments, the researchers used a material called Carbomer—incidentally, a common thickening ingredient found in creams, gels, and lotions. This allowed them to construct a 3D model that replicates the viscosity, stiffness, and porosity of mucus. The 3D material prepared using this innovative approach is also optically transparent, which enables high-resolution visualisation of even single bacterial cells and colonies in 3D space. By further optimizations, the team was able to produce either soft structures with large pores, which imposed low spatial confinement on the bacteria, or, stiff structures with smaller pores for higher spatial confinement. “Using Carbomer to create a 3D growth matrix was a perfect choice because it is synthetic and does not degrade over time. Moreover, we can change the physical stiffness of the matrix without making a significant change in the chemical composition. This meant we could focus solely on the physical environment without worrying about additional chemical factors” explains Dr Tapomoy Bhattacharjee, the principal investigator of the study.
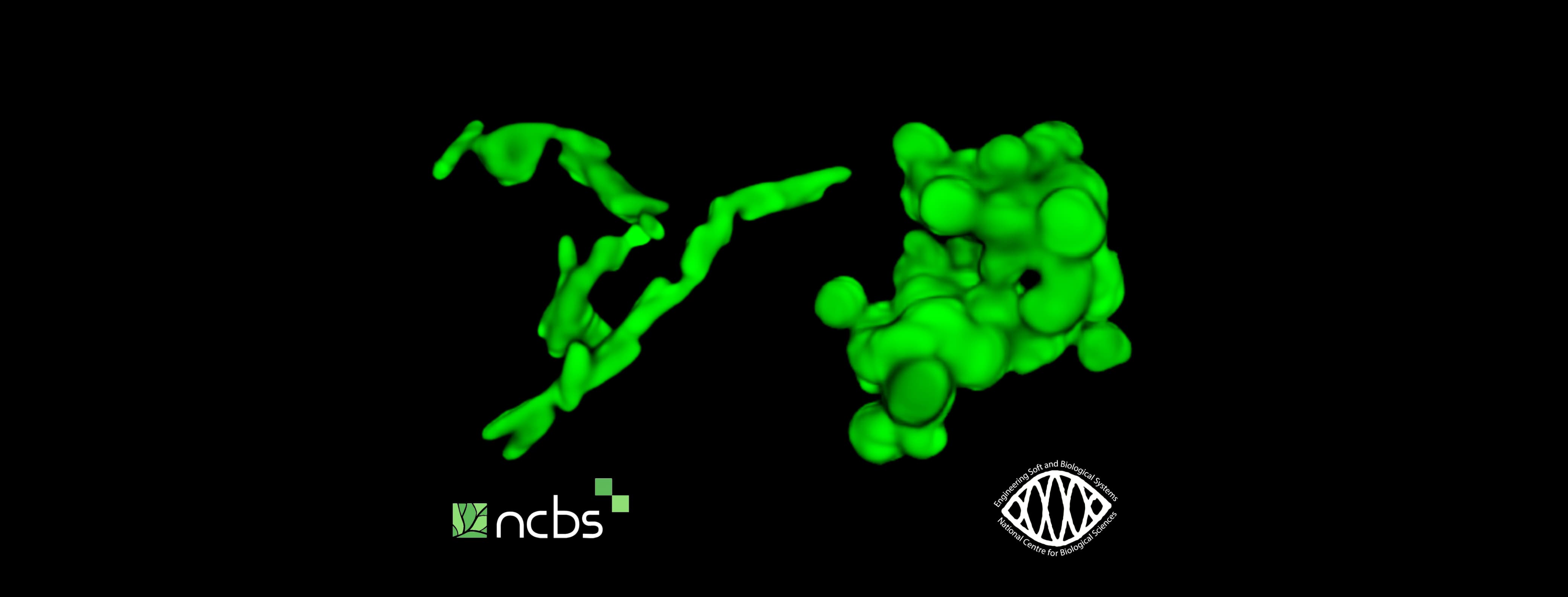
The gut is home to a diverse range of bacteria with varying shapes, sizes, and genetic profiles. To study how either low or high degrees of physical confinement would affect the growth of different bacterial strains, the research team placed them in these 3D models. Interestingly, they observed that regardless of the species, under all conditions of high confinement, rod-shaped bacteria always performed much better than their spherical counterparts. Importantly, when the team made different combinations of rods and spheres directly compete against each other, they observed that rod-shaped bacteria invariably outcompeted spherical ones under high confinement.
Using computational simulations and microscopy to model growth in 3D space, the team found that rod-shaped bacteria enjoy this advantage because they can readily elongate and spread out their progeny during growth, thereby forming colonies with a high surface area and low enclosed volume. However, spherical bacteria form clustered, rounded-up colonies, wherein bacteria trapped in the centre of these compact clusters struggle to access air and nutrients, leading to slower, less efficient growth. This allows the rods to access nutrients more efficiently than the spheres, enabling them to grow better under higher confinement.
“It is an incredibly intuitive insight, really! Think about it like packing together long crayons vs packing together smooth marbles. It is the fundamental difference in the geometrical architecture of individual cells that causes these dramatic changes in growth patterns - which is quite remarkable, because these are effects that you would never be able to capture using traditional culturing platforms” explains Sreepadmanabh.
But what if rod-shaped bacteria somehow possess special genes that give them unique survival advantages? To test this, the researchers turned the rod-shaped bacteria into spherical blobs, without affecting their health or growth rate. And stunningly enough, despite everything except their shape being similar, the spherical bacteria fall far short of matching the growth performance of their rod-shaped counterparts, which validates the idea that cell shape is the determining factor for growth success under higher confinement.
“These findings completely change the way we think about how microbial populations survive and adapt across diverse ecological settings. We show that even beyond the conventional domains of genetic mutations and chemical signalling, there exists a rich class of regulation through the mechanical environment that decides the fate of microbial communities. Our work opens the door for future questions, such as how a range of processes encompassing motility, pathogenesis, adaptive mutations, and evolutionary fitness can all be modulated by the physical constraints imposed by an organism’s surroundings” states Dr. Bhattacharjee, highlighting the broader impact of this study.
Link to the full study here: https://www.nature.com/articles/s41467-024-53989-6








0 Comments