Introduction
This is the second article of our two-part series on historical developments and contemporary research advances in the field of origin of life. In this series’ first article, we provided a broad overview of the questions within the origin of life research, using a historical perspective. We also outlined briefly how inputs, directions and methods from multiple disciplines such as geology, chemistry, and mathematics have been critical in shaping the development of this field. The previous article also described how different theories and hypotheses have been developed over the years for deciphering the origin of life on earth and outlined the need for bridging the gaps between the two major schools of thought in the field: the gene-first and the metabolism-first hypotheses.
In this article, we delve deeper into some of the specific approaches being employed for improving our understanding of the origins of life. We also explore why solving the origin of life problem inevitably requires inputs from a diversity of fields and approaches. In particular, we focus on the research efforts of one such collaborative and interdisciplinary group of scientists working at NCBS Bangalore, University of Delhi and ESPCI Paris, and how they have been collectively studying autocatalysis as a mechanism, which served as an essential pre-requisite for the emergence of complex biomolecules necessary for life.
Need for an interdisciplinary approach
Understanding how the phenomenon of life originated on earth has been one of the most fundamental questions that continue to evade scientists to date. Different experts from a range of disciplines have approached this problem but have met with limited success. Not surprisingly, the need for using a diversity of disciplinary insights and approaches for unravelling such a phenomenon has become increasingly obvious over the years.
Research on the origins of life is heavily dependent on insights from various disciplines, including chemistry, biology, geology, physics, and mathematics to name just a few. “Not only do you need different types of experimentalists, but you also need different types of theorists to work together. And this is a big challenge for the field to manage to bring all those people with a different culture”, explains Dr Philippe Nghe, a researcher at ESPCI Paris who is part of one such collaborative team working to solve this problem.
Each of these diverse disciplines brings unique perspectives and provides different approaches for moving towards a better understanding of the subject. For instance, inputs from geologists help better gauge the geological conditions of early Earth, including its atmospheric makeup and mineral composition, and also help decode a diversity of historical evidence collected from fossils such as stromatolites. Similarly, inputs from biochemistry offer a better understanding of biomolecules such as DNA, RNA, proteins and lipids, and how interactions between them could have led to the emergence of life on Earth.
In addition to such experimental fields of study, insights and contributions from theoretical fields like mathematical modelling and computational biology have also proven to be critical to our understanding of the origin of life and will continue to play an important role going forward. Adopting such an interdisciplinary and collaborative approach also allows more diverse perspectives, data and methodologies to come together in unique ways to shed light on specific aspects of the origin of life on Earth.
One such interdisciplinary team of scientists at NCBS, DU and ESPCI Paris have been collaborating for several years to collectively explore the mechanisms and roles of RNA-based autocatalytic systems in enabling the origin of life. Before we explore this collaborative research project in greater detail, let us first understand a little bit about the theory of Autocatalytic Sets (ACS).
Understanding autocatalytic sets
A catalyst is a substance/molecule that does not get used in the reaction itself but speeds up the reactions between these molecules. Autocatalytic sets (ACS) are collections of such catalytic molecules and chemical reactions capable of producing the entire set collectively. In an autocatalytic set, every member is the product of at least one reaction catalysed by at least one other member of the set. This property allows them to be closed and self-sustaining systems, since every member of the ACS can be produced internally. An ACS is either capable of generating its own food molecules or is dependent on food molecules that are readily available in the environment. This property of self-reproduction is a critical feature exhibited by all living systems.
A simple example of such an ACS in terms of a chemical system would be a set containing two species of chemicals that catalyse each other’s production. Consider the reactions in Figure 1. below. Here P catalyses the production of Q and Q catalyses the production of P. Such a set can be represented as a two-cycle (as shown in the figure below) where the direction of the arrow indicates which species is catalysing which species. P and Q together form an autocatalytic set. As a result of this, both P and Q sustain each other’s production giving rise to the property of self-reproduction.
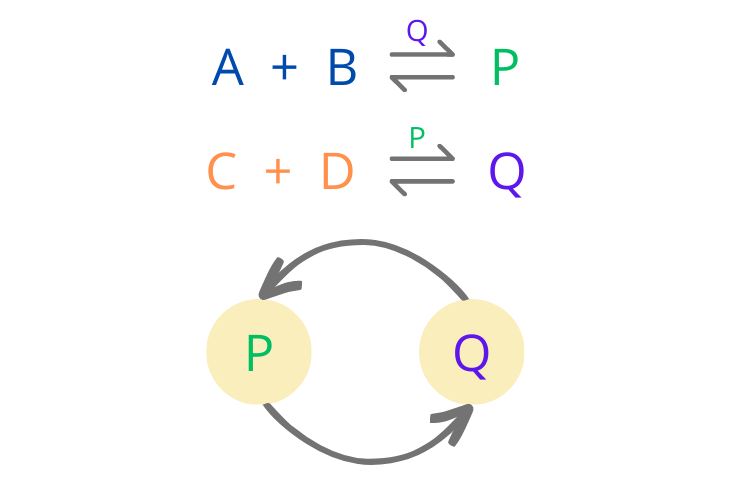
Figure 1: An example of a simple autocatalytic set, where A, B, C and D are the reactants that have been assumed to be available in unlimited supply, while P and Q are the products that are constantly being catalysed by each other.
On a similar note, a cell can be considered a highly complex autocatalytic system capable of self-reproduction. For example, a simple bacterium grows by converting food from the environment and is able to make more copies of itself. It achieves that by doubling each and every component that it is composed of and then dividing it into two daughter bacteria.
This is similar to how a terrarium also works, which is a miniature garden system fully sealed within a closed glass container, which includes plants, water as well as gases. These plants use the water to grow and also create moisture that restores the water in the terrarium. The gases, oxygen and carbon dioxide, are also balanced in this system due to the processes of respiration and photosynthesis occurring in these plants. All of these cyclical processes make the terrarium self-sustaining and self-sufficient.
In 1971, Stuart Kauffman, Manfred Eigen, and Otto Rössler were the first to independently introduce the concept of autocatalytic sets to the origin of life problem. In particular, Kauffman used theoretical arguments supported by computational modelling to show that it was possible for autocatalytic sets of proteins to emerge in early earth conditions. While there have been several theoretical studies of autocatalytic systems over the years, experimental approaches have only gained prominence in the last few decades. Such experimental approaches can enable the building of different model systems for studying the role of lipid-based, DNA-based, and peptide-based autocatalytic chemistries in the origin of life.
Overall, the theory of ACS provides us with a way to think about how chemicals on the early earth could have been possibly organised before becoming complex enough to produce life. However, it is not immediately clear if ACS has the ability to evolve. Over the years, this has fuelled several heated debates questioning whether ACS are actually capable of undergoing Darwinian evolution and increasing in complexity to an extent that can give rise to early lifeforms.
While, experimental studies of ACS have successfully demonstrated self-reproduction, other funda-mental properties of Darwinian evolution such as variation, heredity and selection have received minimal attention from researchers so far. In this context, a recent review by Ameta et al. (2021) elucidates the various advances and challenges of demonstrating Darwinian evolution in autocatalytic chemical systems.
Further, the collaborative group at NCBS Bangalore, the University of Delhi and ESPCI Paris are investigating an experimental autocatalytic chemical system for better understanding properties like self-reproduction, heredity and variation, which are required to explain Darwinian evolution. In the next section, we will dive deeper into this collaborative group’s efforts to study the Darwinian evolution of autocatalytic chemical networks.
Collaborative efforts at NCBS, DU and ESPCI
This collaborative research effort is being carried out at National Centre for Biological Sciences (NCBS), Delhi University (DU) and École supérieure de physique et de chimie industrielles de la Ville de Paris (ESPCI). The group at NCBS includes Dr. Sandeep Ameta, Nayan Chakraborty on the experimental front, who are working with Dr. Shashi Thutupalli, while Dr. Yoshiya Matsubara is studying the theoretical aspects of the project along with Dr. Sandeep Krishna and Dr. Shashi Thutupalli. At DU, Angad Yuvraj Singh is studying the theory of autocatalytic chemical dynamics under the supervision of Dr. Sanjay Jain. And at ESPCI, Dr. Cyrille Jeancolas and Camille Lambert are using both experimental and modelling-based approaches to study the Azoarcus system under the supervision of Dr. Philippe Nghe.
Here, Azoarcus refers to a nitrogen-fixing bacteria, where one of its self-splicing introns also functions as an RNA enzyme (aka ribozyme). The collaborative group at NCBS, DU and ESPCI also uses a model system based on one such ribozyme that is roughly 200 nucleotides long. It was first studied by Hayden and Lehman in 2006, where they demonstrated that it could self-assemble through reactions between smaller fragments in an autocatalytic manner. Later studies also introduced mutations in the sequence of the Azoarcus ribozyme that were manipulated to form autocatalytic reaction networks exhibiting collective reproduction properties.
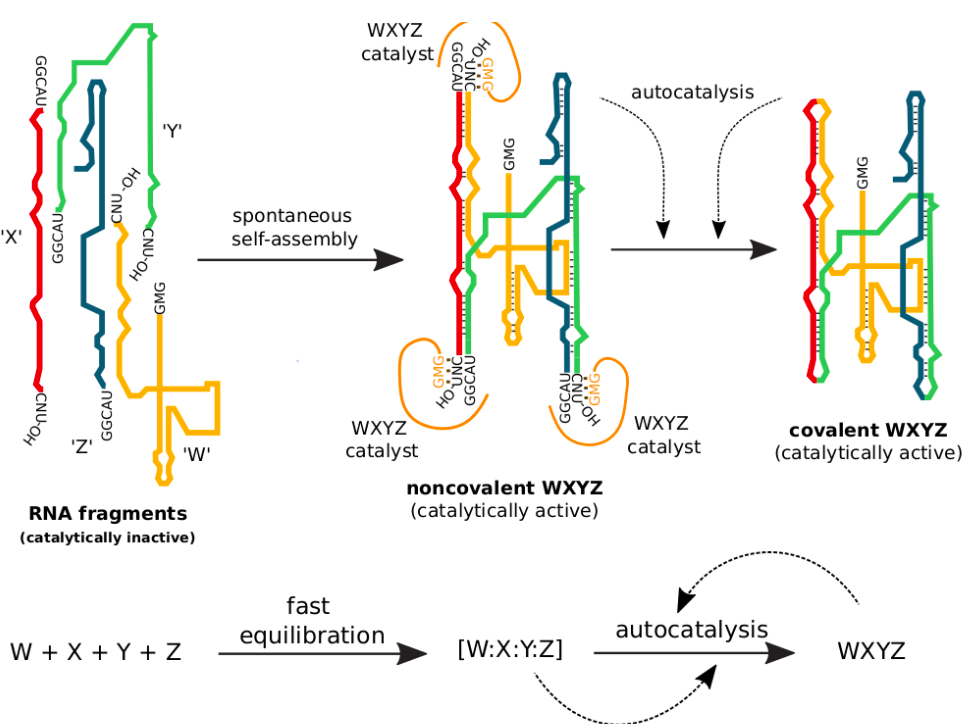
This Azoarcus RNA model system is now being studied by the collaborative group to demonstrate the evolvability of such autocatalytic networks. One experimental setup to demonstrate evolvability is a serial dilution protocol, wherein a small fraction of the RNA networks are transferred (called seeding) from one reaction pot to another having fresh food mixtures. This process generates RNA populations in each reaction mixture (called generations), which are further analysed through genetic sequencing. One of the characteristics of evolution, heredity, can be observed when seeding the same food mixture with two different networks leads to the generation of two different RNA populations. In such cases, the observation of heredity here can be attributed to the autocatalytic nature of these reactions.
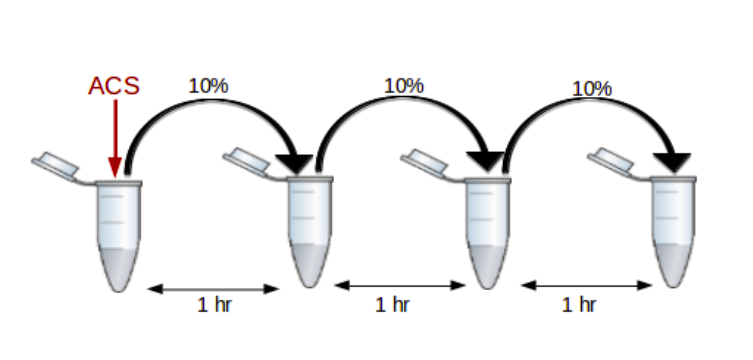
The collaborative group is also investigating other aspects of the evolvability of such RNA autocatalytic networks. They begin by mutating the model system to generate a pool of ribozyme variants. These mutant ribozymes are then subjected to various kinds of selection pressures such as serial dilutions and temperature fluctuations and then analysed through genetic sequencing. In a separate experimental setup, these ribozymes are compartmentalised inside coacervates to study ribozyme reproduction, and eventually their growth and division.
Overall, the team aims to theoretically understand, experimentally demonstrate and computationally model the occurrence of Darwinian Evolution within such autocatalytic sets. One of their key objectives is to demonstrate the very first functional ACS-protocells, i.e. compartmentalised autocatalytic self-reproducing systems with heritable variations exhibiting selection (also called, differential fitness).
“When you talk about Darwinian evolution, not only [do] you need all those properties, but you also need them to work together properly”, explains Philippe. Demonstrating this experimentally could perhaps be the very first documented evidence of a protocell undergoing Darwinian evolution, and thereby the first demonstration of life in the lab.
The collaborative group also continues to push the boundaries of what is known about the origin of life on earth in smaller sub-groups. For instance, Sandeep Krishna and Yoshiya Matsubara are together working on theoretical aspects and modelling of heredity in autocatalytic systems.
Explaining the need for theory and modelling in such efforts, Yoshiya explains that “as theorists, we are focused on developing kinetic models of autocatalytic chemical systems (ACSs). Through these models, we try to identify the necessary conditions, such as properties of chemical systems and experimental protocols, for the emergence of self-reproducing and evolvable ACSs. This will not only help experimentalists in demonstrating Darwinian evolution in the laboratory but also could contribute to making the debate on the origins of life scenarios, like the metabolism-first or gene-first scenario, more quantitative and concrete, and thereby lead to a better understanding of chemical evolution process."
Moreover, using the Azoarcus RNA system, Sandeep Ameta has been experimentally investigating several aspects of variation and heredity in vitro. Shashi Thutupalli and Nayan Chakraborty are investigating the dynamics of ACS in compartments. Further, Philippe Nghe and his group are now investigating ways to identify autocatalysis in a system of diverse chemistries, and particularly developing ways to discover and engineer novel autocatalytic systems. Sanjay Jain and Angad Yuvraj are working on alternate mechanisms to achieve evolution in autocatalytic chemical systems.
Conclusion

Figure 4: The team of researchers across NCBS, DU and ESPCI collaboratively working on origin of life research
Our article demonstrates how interdisciplinary collaborations can seamlessly blend insights from theoretical and experimental fields to provide us with a deeper understanding of the origin of life.
Specifically, systems containing ACS provide us with a framework to understand the prebiotic origins and evolution of the first living cells. Moreover, interdisciplinary investigations in fields such as astrobiology can also shed light on alternate chemistries capable of sustaining life on other planets.
“I think we're at the point where a spurt of "growth" will happen in our understanding. This is mainly due to the new experimental systems that people are now studying, ranging from "messy chemistries" to multiple schemes for autocatalysis, to new mechanisms that generate a diversity of RNA or DNA sequences”, explains Sandeep Krishna.
Despite all the recent advances in our understanding of the processes involved in the functioning of living systems, their biochemistry, morphology, and metabolism, we still lack an understanding of the design principles behind these processes and the underlying phenomena that have given rise to their universality across all organisms.
Some of the most fundamental questions that remain unanswered today include the open-ended evolution of chemical systems, the origin of genetic code, the rise of multicellularity, as well as the emergence of complex traits in living systems such as brain development (cephalization) and cognition. Sandeep Ameta further adds that it’s really crucial to question if “we understand the design principles enough to be able to build synthetic chemical systems with life-like properties”.
We are still far away from conclusively answering fundamental questions about living systems and most of these questions redirect us to the question of “How did life originate?”. Using interdisciplinary and multi-team collaborative efforts (such as that at NCBS-DU-ESPCI) is perhaps the only way to arrive at a holistic understanding of life’s origins.










0 Comments