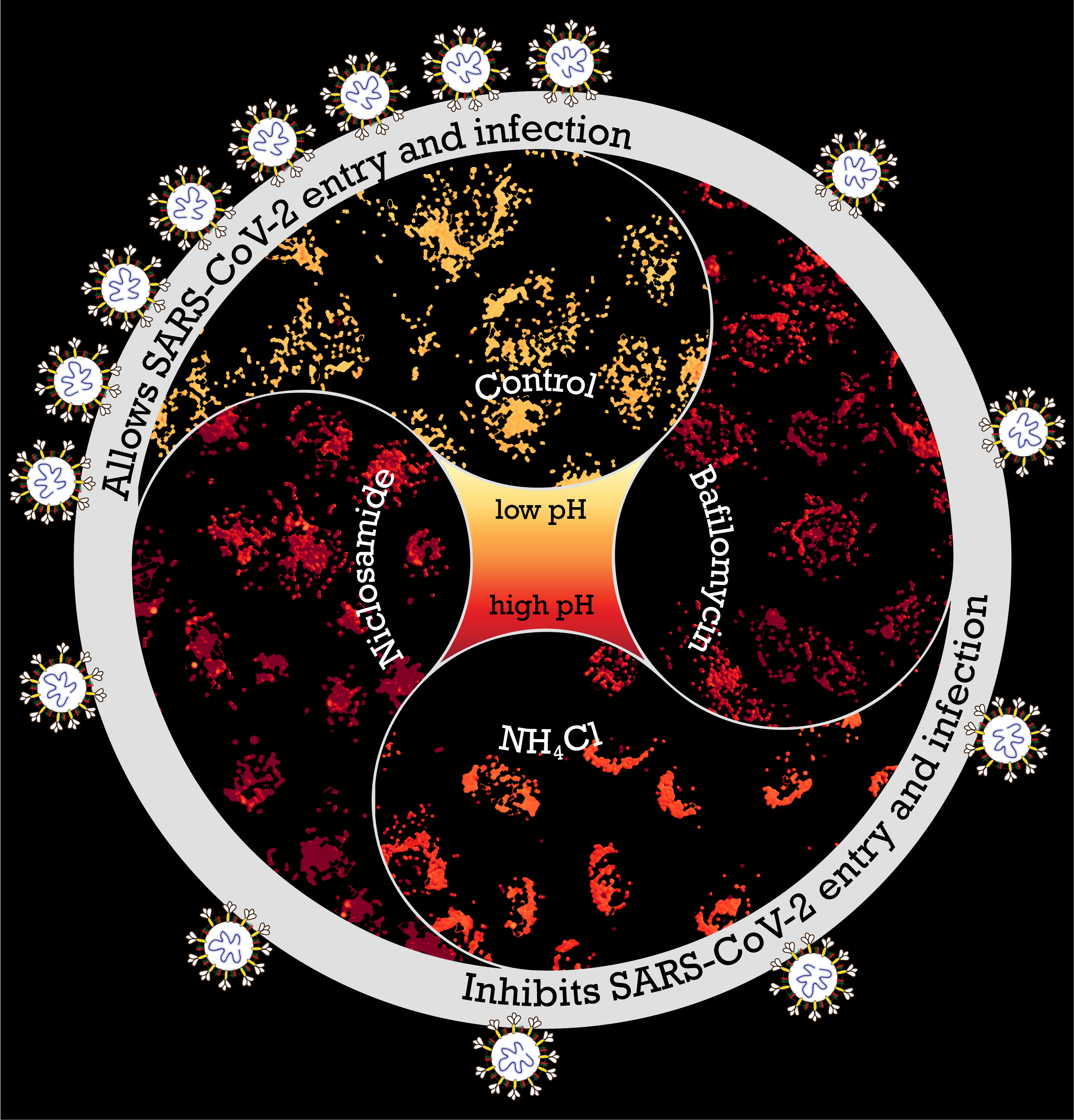
Endosomal acidification inhibitors target SARS-CoV-2 entry and infection. Image by Chaitra Prabhakara; Design by Chaitra Prabhakara and Sowmya Jahnavi.
Cell biology has been a theme of research at the National Centre for Biological Sciences (NCBS) and Institute for Stem Cell Science and Regenerative Medicine (DBT-inStem), since their inception, with fascinating fundamental research questions asked in all the labs under this umbrella. In the wake of COVID-19, there was a pivot in the focus of several labs. Collaborations were born to apply the hard-won skills, techniques and knowledge from various labs to study the viral infection process and potential therapeutics.
2020 saw the start of a large inter-institutional effort between labs on the campus, and the Indian Institute of Integrative Medicine (CSIR-IIIM), fuelled by the desire to combine the expertise of diverse scientists towards alleviating the global challenges of the pandemic we continue to face. In this effort, a large team came together to identify entry mechanisms of SARS-CoV-2 into cells and strategies to inhibit the infections by targeting those mechanisms.
The receptor binding domain (RBD) of the infamous spike protein of SARS-CoV-2 attaches to specific receptors in cells; this has been established in multiple COVID-19 studies. However, the cell entry route of this virus is largely unknown. Identifying these entry routes aids in designing therapeutic strategies. Addressing this, a research article published recently in PLOS Pathogens probed factors that control viral entry into gastrointestinal cells. The mechanism of entry into the cell or “endocytosis” happens through the formation of an “endosome” or vesicle from the cell membrane. Investigating the pathway of entry showed them a requirement for viral entry: the acidified endosomes. This led them to the identification of agents to inhibit acidification of endosomes and thus affect viral infection.
Tackling multiple questions within the study, the scientists involved had to extensively discuss the best way forward when there was limited information about the virus. The experience the Mayor, Guha, and Sundaramurthy Lab scientists have with studying the endocytic pathway formed the foundation of the project, and the experimentation evolved from there, through a period of trial and error for everyone involved. The first authors of the paper are Chaitra Prabhakara, Rashmi Godbole, Parijat Sil, Sowmya Jahnavi, and Shah-E-Jahan Gulzar, who led three themes of the investigation. Chaitra says, “Most of the project was driven by the discussions we all had together, and that was crucial in experimental design and interpretation of the data, which led us to the next set of experiments.”
The experiments began with the simplest probe, and the probes’ complexity grew. With their experience in endocytic work, Chaitra and Rashmi focussed on the cell entry process using the receptor binding domain (RBD) of SARS-CoV-2 spike protein. They found that both endocytic pathways utilised by the RBD (with and without ACE2 receptor) converge on acidified endosomes, this therefore may be a requirement for virus entry. “These experiments gave us a lot of information on the viral and host receptor interactions and diverse endocytic processes employed for cell entry. There’s more information coming out about the gastric cells being a potential host for the virus, so it was a good way to start understanding those interactions,” says Rashmi.
The pseudovirus utilised by the team mimics the entire spike protein of SARS-CoV-2, but does not replicate itself. It is a model that enabled Jahnavi and Parijat to carry out multiple experiments on the process of viral entry into the cell and track infections without using the infectious particle directly. They also used this pseudovirus to test ways to inhibit viral entry. The experiments with the RBD protein and pseudovirus entry into the cells offered many insights, including controlling the acidification of the endosome to prevent viral entry. Parijat explains, “This is a classic example of how fundamental cell biological understanding feeds into making choices for clinical research. We lived through that while doing this project together.” Then it was time to validate their initial findings using clinical samples of SARS-CoV-2.
Jahnavi adds, “Team discussions were important as many of the experiments evolved on the go. It was pretty common to see Chaitra and Rashmi figuring out stuff on the whiteboard.” One of the aspects the team had to experiment in uncharted territory was with drugs that could be repurposed easily. Chaitra says, “We tested a small subset of drugs that are known to affect acidification in other contexts.” The drug they identified for its strong inhibitory effect on the cellular entry of RBD protein and pseudovirus was Niclosamide, commonly used to treat people infected with tapeworms. This was obtained in sufficient quantity and purity from long standing collaborations of one of the groups (Mayor) with Ram Vishwakarma and his colleagues at the CSIR-IIIM, Jammu. They sent almost all the drugs in their pure form very promptly, during the lockdown period.
The testing of the efficacy of Niclosamide and other inhibitors on the real SARS-CoV-2 was possible once approvals and transfers were in place for clinical samples to be used in the investigation. Shah-E-Jahan worked with a few other researchers to test the findings from the pseudovirus against SARS-CoV-2 assays. She says, “It was all new to us and it took some time to standardise and optimise everything. We learned a lot together, it was a nice experience.”
The discussions starting in March 2020 bore fruit in just a little over a year, initially in the form of an open access preprint. Subsequently, evidence of the anti-inflammatory property of Niclosamide was described by a team of scientists in the UK. Along with the independent findings of the Bangalore Life Science Cluster and CSIR-IIIM team of the drug acting as a potential SARS-CoV2 entry inhibitor—blocking the viral entry through pH dependent endocytic pathway— these have prompted immediate clinical testing. CSIR in collaboration with Laxai Life Sciences initiated Phase-II clinical trials of Niclosamide in June 2021. This is a multi-centric, randomised, open-label clinical study to evaluate efficacy, safety, and tolerability of Niclosamide for the treatment of hospitalised COVID-19 patients.
Dr. Varadharajan Sundaramurthy shares his appreciation for the entire team, “This work had a lot of moving parts when we started, and as we were going ahead. The way it all came together is only because of the team effort, and the willingness of the people involved to pause their own projects and concentrate on this as a direct response to the pandemic.” Prof. Satyajit Mayor, echoes this, “The collaborative effort is the really special part of this research. The reason it was possible was because our scientists were able to capitalize on their years of training and expertise and pivot to address the need of the hour. Without this investment over the years and the collaborative spirit, such an effort would have taken a lifetime to put together from scratch.”
The work began as the nation was under a lockdown, and the campus came together to support this COVID-related work, from sharing reagents, to administrative support and more. Chaitra and Jahnavi refer to other sources of encouragement, especially from other scientists: knowledge and reagents were shared happily across the world. The diverse laboratory and analytical skill sets of all the scientists involved in this work were crucial to bring it together. Rashmi speaks to the team’s sense of purpose and satisfaction, “It was good to see that we could apply some cell biology, and identify a drug that can be repurposed in such a short span. It was a Herculean task if one of us had to do it, but we pulled it off together.”









0 Comments