For an avid exerciser, a muscle pull or tear is a painful and fairly common occurrence. A sudden turn or an unusually vigorous bout of aerobics can leave one with a muscle tear that will effectively confine a person to bed for a few weeks. However, muscles do heal - a set of quiescent cells called myosatellite cells in muscles are activated by injury to divide and form myoblasts, which in turn fuse with muscle cells to repair damaged muscles. The mechanistic basis of myoblast fusion with muscle fibers is now clearer thanks to recent work from Vijayraghavan's group at the National Centre for Biological Sciences (NCBS).
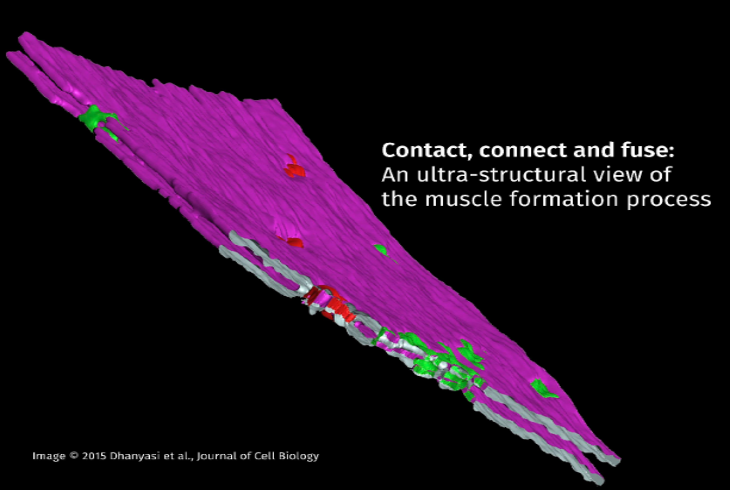
Nagaraju Dhanyasi from Vijayraghavan's group, has collaborated with Prof. Ben-Zion Shilo and Dr. Eyal Schejter at the Weizmann Institute of Science, Israel, who are also investigating the processes governing myoblast fusion in flies and mice. To study the process at a high resolution, it was necessary to generate electron microscope (EM) images. This allowed researchers to reconstruct the steps in the dynamic process of myoblast fusion, where the structure of the fusing membranes was closely examined. The work involved molecular biology and state-of-the-art electron microscopic techniques, which were carried out in collaboration with the EM facility of the Weizmann Institute. The team's investigations have resulted in a description of the events during the merging of individual myoblast cells with muscle cells, and was published in the October issue of the Journal of Cell Biology.
Most of our knowledge about the fusion of myoblasts to form multinucleate muscle fibers has been gleaned from studies on the development of tubular muscles of Drosophila larvae. However, the mechanisms governing myogenesis (formation of muscle fibres) in striated muscles of the skeletal musculature in vertebrates is not very clear. This study focuses on the events occurring in myoblast fusion in Drosophila during the formation of flight muscles. These muscles serve as a particularly attractive model, since their developmental program, and their muscle fibre organisation resemble key aspects of vertebrate skeletal myogenesis.
"This work is actually a continuation of studies carried out by Vijay's earlier students - Priyankana Mukherkjee and Rajesh Gunage", says Nagaraju Dhanyasi, the first author of the paper. "Priyankana began this work by investigating the role of various fusion proteins using confocal microscopy in the myogenesis of flight muscles of Drosophila. This work was also a collaboration with Drs. Shilo and Schejter. Rajesh established the presence of stem cells in Drosophila muscles similar to the satellite cells in vertebrate muscles. This set the stage for addressing questions on vertebrate muscle regeneration in a Drosophila model system." The advantages of using Drosophila as a model are many - the tiny fly is easily grown and is amenable to a huge array of genetic manipulations. In spite of this, the study of myogenic processes in flight muscles has lagged behind, primarily because tools for the genetic manipulation of later developmental phases were lacking. "With the advent of RNAi technology, this problem was solved, and we were able to study the myoblast fusion process in great detail", says Dhanyasi.
The study shows that the fusion of myoblasts to existing muscle fibres, called myotubes, follows a set of distinct stages that requires communication between transmembrane elements and the actin cytoskeleton. An elegant series of experiments allowed the researchers to delineate the ultra-structural details of a series of discrete steps in this event. The process begins with myoblasts binding to the surface of an existing myotube with the help of a host of cell adhesion proteins, a process known as apposition. Following apposition is a flattening of the myoblast membrane to increase its contact surface with the myotube. This step has been shown to require elements of the cell cytoskeletal machinery. The third step in this process is a crucial one where the myoblast membrane and the myotube surface are brought very close to each other in a condition known as 'tight apposition'. The tight apposition forms multiple areas of cell-to-cell contacts called 'nascent pore sites'. These contact areas form fusion pores, where the cell membranes of the myoblast and myotubes merge. The fusion pores eventually expand until the myoblast is fully incorporated into the growing myotube.
Future studies are likely to involve detailed investigations on the mechanisms of fusion pore formation and in discovering the molecular players involved in pore formation. "We believe that the cellular and molecular mechanisms uncovered in this study, and in future studies are highly conserved, and therefore also applicable in vertebrate systems. This study is therefore likely to provide key insights into understanding muscle development and repair processes", says Dhanyasi.
The paper titled "Surface apposition and multiple cell contacts promote myoblast fusion in Drosophila flight muscles" can be accessed here.









0 Comments