Eric Carl, in his much-loved children's book, "The Very Hungry Caterpillar", takes us through the transformation of a gluttony caterpillar into a beautiful butterfly. For a scientist, however, this book is a Pandora's box of questions. How does the caterpillar know when to stop eating? Had he not eaten so much, would he have ever moved on into the cocoon?
A group of researchers at the National Centre for Biological Sciences, Bangalore, have now uncovered the neuronal typeset that determines a larva's decision to pupariate, especially when challenged for nutrients. The group, led by Prof. Gaiti Hasan has investigated this question in fruit flies to understand how they integrate internal and environmental nutritional cues to make decisions on pupariation.
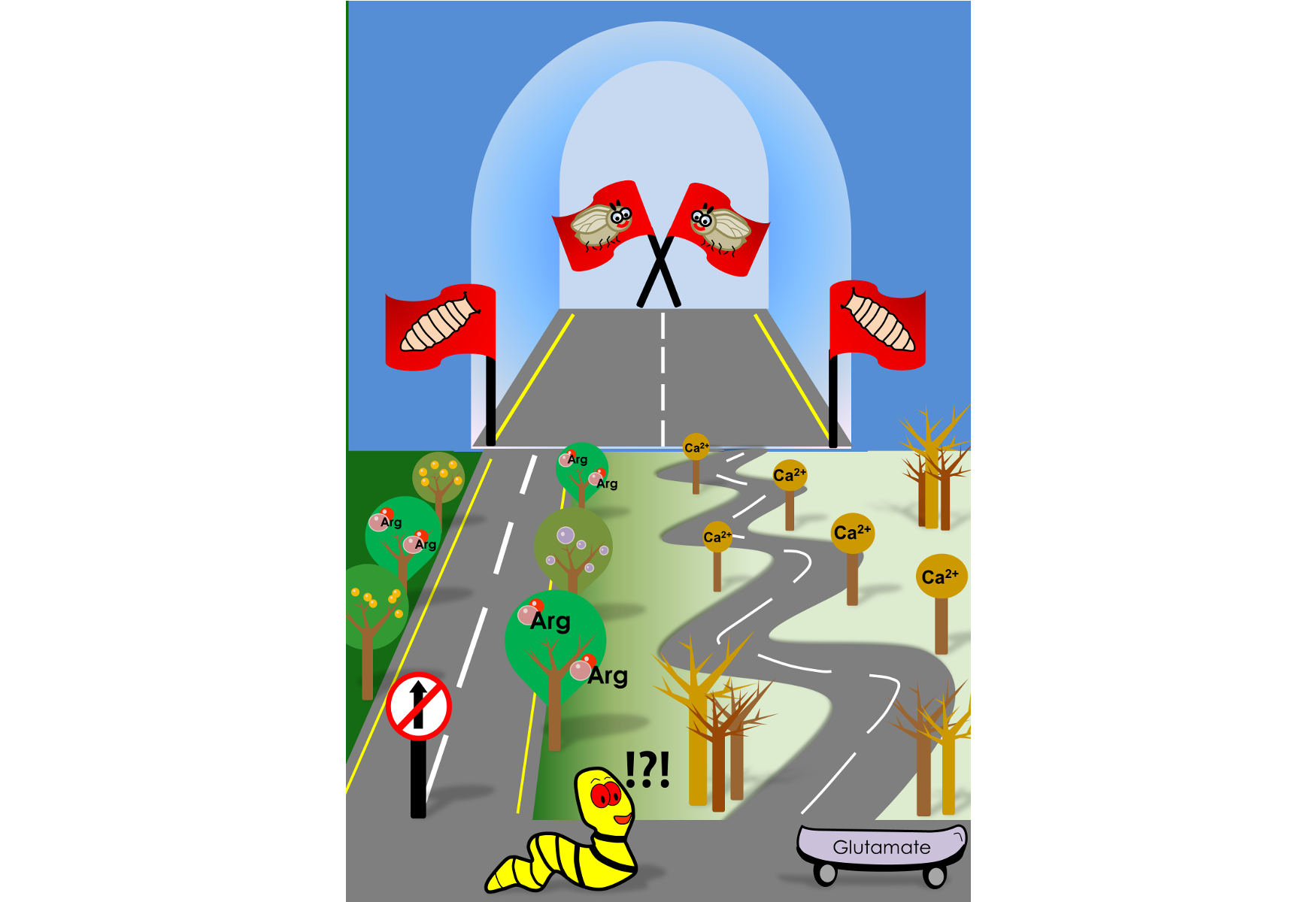
Like many insects, fruit flies go through developmental milestones. Larvae hatch from eggs and feed voraciously until they hit a 'critical weight'. Attaining critical weight gives larvae the license to pupariate, even if they are subsequently starved. However, larvae must comprehend nutrient-poor conditions and make sure developmental processes would not be hampered during pupal stages, a critical phase where the larva prepares to transform into its adult form. This study, published recently in the Journal of Neuroscience, has identified neuronal and molecular switches that signal development under nutrient limiting conditions.
Previous work from the same group had identified a set of neurons in the larval ventral ganglion that regulates pupariation on a protein deficient diet. The ventral ganglion in flies is analogous to the spinal cord in vertebrates. These neurons were found to be glutamatergic in nature, meaning, they made and released the excitatory neurotransmitter, glutamate, to communicate with their neighbours.
One evening, Siddharth, one of the lead authors of this paper, decided to do a fun experiment. He activated specific ventral ganglion glutamatergic neurons and observed that these animals pupariated prematurely, but died as adults. What started off as a casual experiment, turned out to be the springboard for this study.
Convinced that certain glutamatergic neurons of the ventral ganglia were central to this developmental switch, Siddharth decided to test if these neurons could sense the loss of environmental nutrients. By mimicking nutrient deprivation on a Petri dish, Siddharth found that the ventral ganglia glutamatergic neurons during the late larval stages displayed characteristic calcium oscillations. 'Calcium transients' as they are also called, are a typical language for neuronal communication. These calcium transients were however absent in neurons of larvae taken from an earlier stage of development, indicating that such responses were stage-specific.
The glutamatergic neurons are nested deep within the larval neuronal network. How then could they sense loss of nutrients in the external food? This, the authors show, is carried out by cholinergic neurons, a class of neurons that decorate the larval body wall and produce the neurotransmitter, acetylcholine. Specifically, cholinergic neurons sense loss of the amino acid, arginine, with the help of an amino acid transporter called ‘Slimfast’. Using arginine as a proxy, cholinergic neurons conveyed nutritional information to the glutamatergic neurons, resulting in those calcium waves. To make matters more complicated, these glutamatergic neurons not only had receptors to receive cholinergic inputs but were also found to harbor receptors that could capture neuropeptide signals. Other groups have shown that neuropeptides are generated from the brain and other organs upon starvation. The glutamatergic neurons thus integrate nutrient information from the environment and from internal tissues.
What molecules generate these calcium transients, asked Shlesha, another lead author of this paper. Imaging and gene sequencing experiments from glutamatergic neurons revealed that the calcium transients were regulated by ion channels on the plasma membrane, as well as those located within the intracellular compartments of the cell such as the inositol-trisphosphate receptor (IP3R) and a calcium-sensor, STIM. In fact, STIM function was found to regulate the expression of ion channel genes in the glutamatergic neurons, thereby modulating their 'excitability' quotient.
Finally, the authors decided to understand how these calcium waves in glutamatergic neurons signalled the decision to pupariate.
"When challenged with protein deficiency, calcium transients are generated in certain glutamatergic neurons. This serves as a cue for release of insulin-like neuropeptides from neurons located further up in the larval brain. The release of insulin-like peptides is known to push larvae into pupariation", explains Prof. Gaiti Hasan, the group leader.
So, in the absence of food, the hungry caterpillar may still have made it to the cocoon - thanks to this nutrient sensing network in its brain.
This article is based on the original research work titled "A multi-component neuronal response encodes the larval decision to pupariate upon amino acid starvation", published in the Journal of Neuroscience on 9th October, 2018. The lead authors Siddharth Jayakumar, Shlesha Richhariya and Bipan Kumar Deb completed their Ph.D in the lab of Prof. Gaiti Hasan, National Centre for Biological Sciences, TIFR, Bangalore. Currently, Siddharth is Post Doctoral researcher with Prof. Venkatesh Murthy at Harvard University, USA and Shlesha is a Post-Doc in the lab of Nobel Laureate, Prof. Michael Rosbash at Brandeis University, USA.









0 Comments