The Overcharged Heart
In March this year the Indian news media reported the onfield death, by cardiac arrest, of 28-year-old Bangalore footballer D. Venkatesh. Many young athletes die like this every year - recent victims have included stars of the European soccer scene and American basketball. In most cases, these deaths are due to an inherited form of the disease, hypertrophic cardiomyopathy. Basically, the heart muscle gets too big for its own good. Its muscular walls lose the flexibility needed for normal pumping, and when the demand for blood flow jumps too high - most commonly while exercising - the hypertrophic heart cannot keep up. And the problem is not limited to athletes. It can also strike the more sedentary among us.
Scientists at NCBS and inStem, Bangalore, are trying to understand the underlying biology of inherited cardiac hypertrophy (HCM), and also the converse disease, in which the heart muscles thin out (dilated cardiomyopathy, DCM). In each disease, the most common genetic cause is a mutation in one of the proteins that comprise the cellular machinery for muscle contraction (see figure). The net outcome of any individual mutation - either a hyper- or hypo-trophic heart - depends on whether muscle contraction is increased or decreased by the change in the mutated protein. But how that increase or decrease in efficiency is brought about remains unclear.
In a recent paper published in Bioinformatics and Biology Insights, the NCBS-inStem team describe in greater detail than ever before the structure that tropomyosin adopts in the so-called "open" position. When the molecule is in this position, myosin can fully engage with actin and initiate muscle contraction. "This study was an important part of a larger project, where we, Jim Spudich and Henrik Flyvbjerg are studying how the amino acid sequence of myosin affects its structure and function", says R. Sowdhamini, who was awarded the Human Frontier Science Program Grant that funds the work. As was explained to me by Margaret Sunitha, the paper's lead author, the current findings help to explain the types of pathology that are seen in the fourteen tropomyosin mutations that cause HCM (11) and DCM (3).
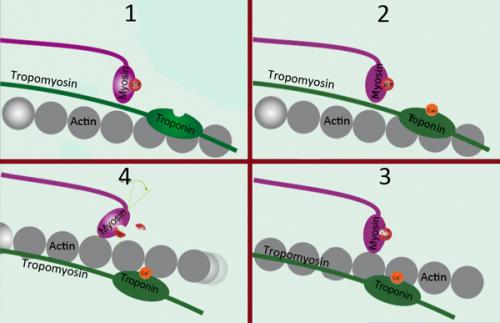
The cyclic nature of heart pumping rests on the alternating ability of myosin to engage with actin. In state 1, tropomyosin blocks the interaction of the two, but after calcium binds to troponin (2), tropomyosin rotates around actin, permitting the myosin 'head' to engage actin (3). Finally, the myosin head tilts back towards its tail, dragging the actin filament with it. Modified from a wikicommons image. Geoff Hyde
To derive their more detailed structure of tropomyosin, and understand its role in contraction - normal and otherwise - the authors pieced together findings from eight different experimental and theoretical approaches. The linear tropomyosin molecule has two cardiomyopathy hotspots along its length. Further, each hotspot has two halves, with opposite roles - one helps myosin and actin to engage, the other keeps them apart. If a mutation is in the first half of a tropomyosin hotspot, the heart cannot contract normally. If in the second, its ability to relax is impaired. In both scenarios, the mutations appear to affect the ability of tropomyosin to position itself normally with respect to the actin molecule.
They note that in nearly all tropomyosin mutations, the amino acid that gets switched in is “charged” rather than neutral: that is, it is electrically positive or negative. This would restrict tropomyosin’s normal precise movements around the actin molecule, much in the same way that two magnets on a table cannot always be moved independently. The substituted, overcharged amino acid of the mutant tropomyosin could be repelled or attracted by any charged chemical residues it encounters in the actin molecule. And that could be enough to disturb the delicate dance tropomyosin performs on the surface of actin during every heartbeat.
John Mercer, one of the inStem members of the team, says: "These are very exciting results that provide important insights. The work done in Dr. Sowdhamini's laboratory is both complementary to and synergistic with our own work, which is biochemical and biophysical". For Margaret Sunitha, who is doing her PhD in Sowdhamini's lab, the project has been very rewarding: "It was a wonderful experience studying the cardiac thin filament structural model. I hope it will open doors and suggest different ways to think about the important roles of these proteins".
And does the work hold out any promise for new therapies in the future? Mercer believes "the findings do raise the possibility of drug treatments that could target inherited cardiomyopathies on a mutation-by-mutation basis".
You can see the paper below, and a video Abstract that explains the findings, by clicking on this link.
Integrative Structural Modelling of the Cardiac Thin Filament: Energetics at the Interface and Conservation Patterns Reveal a Spotlight on Period 2 of Tropomyosin
Margaret Sunitha, John A. Mercer, James A. Spudich and Ramanathan Sowdhamini
Bioinformatics and Biology Insights 2012:6 203-223 doi: 10.4137/BBI.S9798

Comments
Impressive work Margaret !
Molecular biology is
Post new comment