doublesex doubles as gender differentiator and mimicry supergene
doublesex is one multitasker. The gene that controls gender and sexual differences in insects is also a mimicry supergene that determines wing pattern variations in a mimetic butterfly, according to a study led by scientists from the National Center for Biological Sciences (NCBS) and the University of Chicago. The work published in Nature today also redefines the mimicry supergene in this butterfly. For over half a century, biologists believed it to be a cluster of tightly-linked genes, but this study confirms that the mimicry supergene is a single gene. While reporting this discovery and a novel function for a vital developmental gene, "...our study throws new light on the intriguing genetic basis of wing patterning in a butterfly that mimics others," says lead author Krushnamegh Kunte, Reader (Assistant Professor) and Ramanujan Fellow at the NCBS.
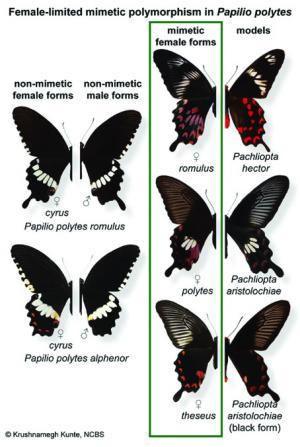
Deception is the name of the gameWing patterns of butterflies have been a hot topic of study due to the diversity of shapes, sizes, colors and markings that they exhibit. But their endearing wing patterns do not make butterflies any less tempting to predators. Butterflies face fairly high predatory inflictions which compel them to go to great extents to ward off their hunters. Some are inherently toxic - predators that eat them once would think twice before attacking another similar individual. To avoid being eaten, the palatable non-toxic species have a deceptive trick up their sleeve: they imitate the wing patterns of toxic butterflies found in the region, a phenomenon known as Batesian mimicry. Often, it is only females that mimic a toxic model (see Figure 1) as they are more prone to predation than males. This has led to sexual dimorphism (marked differences in gender) in many mimetic species. In some cases, females of a single mimic species imitate not one, but many toxic species. This results in many female forms or morphs: what scientists call polymorphism.
The story of the Common Mormon
The Common Mormon (Papilio polytes) is a perfect example of such sex-limited polymorphism. Widely distributed across Asia including India, the species is edible to a wide range of predators. However, Batesian mimicry saves its skin. The male Common Mormon has a single non-mimetic wing pattern but the female has four forms (which lends this species its tongue-in-cheek English name): a non-mimetic morph that resembles the male, and three other forms that mimic the toxic Pachliopta butterflies (the Common Rose Pachliopta aristolochiae and the Crimson Rose Pachliopta hector) found around them (see Figure 2). These females mimic the colors, wing patterns and even shape (note the small tail-like projections on the hindwings) of the Pachliopta models.
This is undoubtedly an impressive natural phenomenon. But how does this work? In the 1960s, two British scientists Cyril Clarke and Philip Sheppard performed initial breeding experiments on various P. polytes populations, which led them to believe that alternative wing patterns were controlled by a supergene. They envisioned this supergene as a cluster of tightly-linked genes located near each other and capable of functioning together as a single genetic unit. Each of the genes in the cluster was thought to control a specific attribute of wing patterning such as the presence or absence of tails, color differences on the forewing, hindwing, and so on.
Questions and testing theories
So which supergene controls butterfly wing patterns? What genes constitute this supergene? How do these genes produce the mimetic morphs? Kunte teamed up with Marcus Kronforst, Neubauer Family Assistant Professor of Ecology and Evolution at the University of Chicago, and other colleagues from Cornell and Boston Universities to find answers to these questions. They had to first determine the potential genes that could be influencing wing pattern variation, a feat they accomplished using genetic mapping. Genes are located on chromosomes, and genetic mapping helps pinpoint their precise positions. To do this, the scientists established pure-breeding (individuals which always pass down a specific phenotype or physical characteristic) lineages of mimetic polytes and non-mimetic cyrus female forms of P. polytes. They generated hybrids between these lineages and then bred hybrid males with females of the genetically recessive phenotype, i.e., the cyrus form. They thus obtained 443 grand-daughters for the cyrus and polytes forms, which they then used for genetic mapping of the mimicry phenotype. "Setting up the right genetic crosses to generate promising mapping broods was pretty challenging," says Kunte. The team used highly-specialized genetic tools and analytic tests to compare the DNA sequences and genomes (the entire hereditary information) of these butterflies. They zeroed in on five genes that seemed to be eligible candidates controlling wing pattern variation.
A gene called doublesex was one of them. Crucial to insect biology, its role in determining sexual differentiation during early insect development is very well-characterized. Surprised at the possibility that this gene could be associated with polymorphic wing patterning, the scientists conducted another suite of tests to determine the role of doublesex in P. polytes. They sequenced genomes of 30 females and compared them against a reference genome. They also examined minute genetic variations between these individuals and analyzed the associations between them. Interestingly, doublesex turned out to be the primary gene associated with mimetic wing patterns, further implicating the pivotal role of the gene in mimicry. "The co-option of this well-known sexual differentiation gene in controlling polymorphic mimicry was indeed an unexpected and very exciting discovery," says Kunte.
doublesex, the mimicry supergene
So how does doublesex double as gender differentiator and mimicry generator? The team discovered that doublesex is a hotspot of molecular evolution: dozens of genetic changes or mutations in the regions that make proteins, and thousands of mutations in other regions of this gene distinguished the mimetic form polytes from the non-mimetic cyrus. However, certain regions of this gene did not show significant mutations while other regions had a dense concentration of protein-altering ones. This may explain how doublesex managed to retain its ancestral function of sexual dimorphism while selectively evolving in P. polytes to control mimetic polymorphism. The team also discovered that more answers lay in the proteins that doublesex could concoct. A single gene can perform different functions if it can manufacture different proteins. Genes accomplish this by a process called alternative splicing, where different portions of a single gene are removed and re-structured to form isoforms. These isoforms produce different proteins which subsequently give rise to unique phenotypes. When the team examined alternative splicing (a well-studied phenomenon in doublesex) in the supergene, they saw that it did alternatively splice into a single male isoform and multiple female isoforms which were expressed at varying levels in mimetic and non-mimetic females of P. polytes. The authors further simulated structures of the doublesex proteins in mimetic and non-mimetic female forms and found them to be substantially different. Wei Zhang, post-doctoral fellow at Kronforst's laboratory and co-first author of the study, applied her advanced bioinformatics skills here. "Differences in protein structure and gene expression among the female forms play a role in making doublesex the ultimate mimicry gene," says Zhang.
But such differences can also be lost during recombination, the usual shuffling of genetic variation at the time of sexual reproduction. To find out how doublesex dealt with this, the scientists re-examined their vast genomic and mapping datasets and discovered that recombination was prevented exactly in the region where doublesex was located. This was enabled by an inversion - a break in the chromosome which causes a small piece to flip and rearrange itself back into the chromosome. Based on these results, the authors argue that a mimicry supergene can be a set of numerous closely-linked mutations in a single gene and not necessarily a cluster of several tightly-linked genes as defined for the past half century.
Summing up, doublesex in P. polytes has retained its function of sexual differentiation while evolving a new function at the same time: a function which permits the expression of many physical differences in just one sex. A startling revelation which, coupled with a revision of what supergenes are, makes the study an important landmark in evolutionary genetics.
That's an observation scientists not involved in the study have been making too. "Not only is this a novel discovery in mimicry genetics, it is more generally important in understanding the multitude of ways that genotypes determine phenotypes in the course of development. This paper is in my opinion an instant classic in evolutionary genomics," opines Lawrence Gilbert, renowned naturalist, ecologist and evolutionary geneticist from the University of Texas at Austin (USA).
Modern technology and hard work pay off
"We would not have been able to map the mimicry gene and find out so many cool things in such a short period of time without modern technological advances," says Kunte. Short is probably a modest understatement, for the results did take quite some time to unearth. "Five long years of sometimes 16-hour work days," he adds. "It is a considerable amount of time, but it could have taken longer if we were not this lucky, and if we did not have such great collaborators working as a team.""Recent advances in DNA sequencing technology have really opened up these classic examples of adaptation to detailed molecular investigation. By sequencing the genomes of many individual butterflies with different wing patterns, we were able to fairly quickly zero in on the one gene that seems to be the critical switch mechanism," says senior author Kronforst.
Apart from the exciting results it revealed, the study has given rise to quite a few queries about wing pattern evolution. Kunte's team at NCBS is currently investigating the evolution, genetics and development of butterfly wing patterns both within the lab and as part of a multi-institutional collaborative team. "How diverse are natural selection and the molecular genetic mechanisms that produce these contrasting wing patterns? How have the genes evolved? The possibilities are endless, and we are looking forward to answering these questions all the way from field ecological to molecular genetic studies."
The authors hope to examine similar instances of polymorphism in other butterflies to determine whether doublesex or other genes act as mimicry supergenes. In some Papilio species, genes other than doublesex control female-limited mimetic polymorphism. What makes members of the same genus so different? "Papilio is an exceptionally diverse genus both in terms of species and morphological diversity. Its evolution has probably been shaped by over 50 million years of ecological and social pressures such as mimicry and sexual selection. This must have deeply influenced their wing patterns, genetics and development. Some species are monomorphic, some sexually dimorphic and others polymorphic," says Kunte. "I expect this combination of varied levels of polymorphism and diversity of color patterns to have divergent genetic bases. The interesting quest in the long term may be to find out ecological generalities that give rise to specific genetic architecture of wing patterns. This is a long road, but an exciting one."
Citation: Kunte, K., Zhang, W., Tenger-Trolander, A., Palmer, D.H., Martin, A., Reed, R.D., Mullen, S.P. and Kronforst, M.R. 2014. doublesex is a mimicry supergene. Nature, Published online 5th March 2014. (DOI: 10.1038/nature13112)
Read the commentary on the paper published in the same issue of Nature here.





Comments
Post new comment