Blue fluorescence lights way to easier stem cell biology
Better and more efficient laboratory techniques are a dream come true for any scientist. That dream could materialize for stem cell biologists thanks to a new finding by NCBS's Mitradas Panicker and his team that makes one of the most important steps in stem cell research - that of identifying human pluripotent stem cells (cells that can differentiate into any kind of adult cell) - easier. The scientists show that a blue fluorescence is emitted by fat-storing organelles or lipid bodies in specific types of pluripotent stem cells. They utilized this to distinguish these stem cells from others more easily and efficiently. The new technique is also a cost-effective and less labor-intensive alternative for scientists who need to culture stem cell colonies for research experiments. Scientists are hopeful that delving deeper into the principles of this technique could throw more light on pluripotent states and the process of pluripotency itself, a significant step towards understanding stem cells better.
Stem cells are undifferentiated cells that can differentiate into cells that are specialized in form and function. They are the crux of the contemporary field of stem cell biology, with which scientists have discovered novel stem cell applications ranging from drug testing to organ repair, transplantation and regeneration. Pluripotent stem cells (those that differentiate into any kind of adult cell) occur naturally in embryos. Two kinds of pluripotent stem cells which arguably represent different developmental states can be isolated from them. The inner cell mass of mouse embryos before implantation on the uterine wall provide naive-state pluripotent stem cells; and post-implantation mouse embryos generate primed-state cells, which are thought to be more developed than naive state cells. Interestingly, pre-implantation human embryos give rise to primed state pluripotent stem cells when cultured in the laboratory. Pluripotent stem cells can also be induced by genetically reprogramming mouse and human somatic cells. The induced pluripotent stem cell counterparts display the same differences seen between mouse and human embryonic stem cells. Induced pluripotent stem cells (iPSCs) are developed in labs and used to derive cell lines and large numbers of cells for experiments.
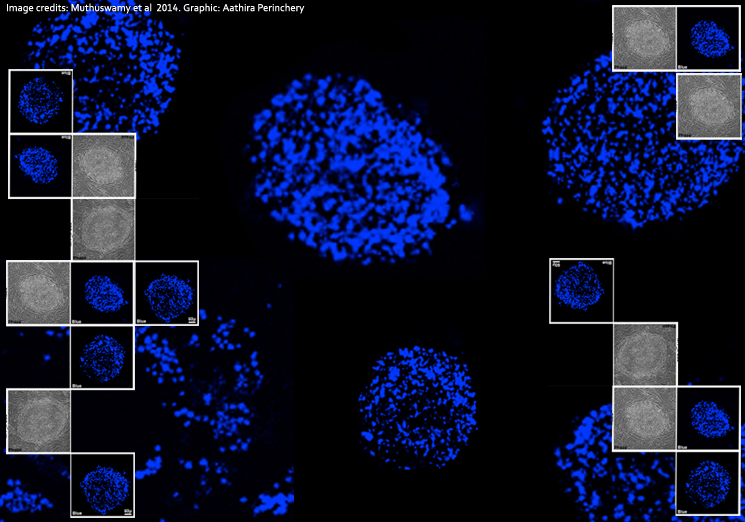
Culturing human pluripotent stem cells (HPSCs) however, is fraught with challenges. Constantly-occuring cell differentiation makes it difficult to maintain cultures with just pluripotent cells. Identifying and isolating HPSCs from a melange of differentiated and undifferentiated cells is therefore a necessity and a fundamental aspect of stem cell research. Existing techniques such as manual isolation based on morphology and antibody-based staining for stem cell markers (genes and protein derivatives that can be used as labels to identify cells) have to be followed by sorting for pluripotent stem cells. These methods are labor-intensive, time-consuming, expensive and sometimes erratic in efficiency. These considerations and a chance observation of an innate blue fluorescence from HPSCs during experiments at NCBS faculty Mitradas Panicker's lab raised a question: could the fluorescence be related to pluripotent states? Can this fluorescence be applied to identify and isolate HPSCs?
Initial laboratory experiments indicated that the fluorescence emerged from lipid bodies, small spherical structures close to the cell's nucleus. A recent fluorescence lifetime-based microscopy technique (which works on the principle of a decay rate, the average time taken by a molecule to re-emit a portion of light that falls on it) used the differences between the ratio of blue fluorescence from cellular components such as NAD(P)H and fat-storing lipid bodies to identify HPSCs. This confirmed the team's observations: lipid bodies were emitting blue fluorescence. Yet, the reason for the fluorescence still remained speculative.
To find out more about the fluorescence, Panicker and his team comprising Thangaselvam Muthusamy, Odity Mukherjee, Radhika Menon and P.B. Megha isolated and characterized lipid bodies from human embryonic stem cells. A detailed analysis of lipid bodies showed that the fluorescence arises from retinyl esters sequestered in these structures. Exploring further, the scientists saw that cells containing fluorescent lipid bodies also tested positive for human pluripotent stem cell markers. The characteristic blue fluorescence could therefore be used to identify and isolate human embryonic and induced pluripotent stem cells. The scientists realized that determining the ratio of blue fluorescence of NAD(P)H to that of lipid bodies using fluorescence-lifetime measurements and very sophisticated microscopy was in fact unnecessary.
After developing this novel technique to identify and isolate human embryonic and iPS cells, Panicker and his colleagues established procedures to sort human pluripotent stem cells from cultures using flow cytometry. At the NCBS Flow Cytometry Facility, they employed fluorescence-activated cell sorting (FACS), which separates cells based on the fluorescence they emit. They independently stained cells to check for pluripotency and to determine if this was linked to blue fluorescence. They also looked for the presence of fluorescent lipid bodies in mouse embryonic stem cells using imaging techniques and lipid body-specific staining procedures.
That was when the scientists realised that the blue fluorescence was specific to only primed human pluripotent cells, and not to the naïve mouse pluripotent cells. This proved interesting since they now also had a novel marker to distinguish between primed and naïve states. "The fact that fluorescence not only identifies pluripotent stem cells but marks state-specific pluripotent cells (primed vs naïve cells) is a very exciting finding," says Odity Mukherjee, lead author and a research scientist with the Panicker Lab at NCBS.
Inspired, the team converted naïve mouse and primed human pluripotent stem cells to their respective alternate states (primed and naïve) to see if fluorescence patterns changed. Indeed, human somatic cells acquired blue fluorescent lipid bodies very early during reprogramming to the induced pluripotent state. Thus, blue fluorescence is an early sign of reprogramming of human somatic cells, and can be easily utilized as a marker to track cell differentiation.
Panicker and his team are excited about the implications of all these results. And rightly so, for their new method is faster, easier and a more efficient technique to identify, isolate and propagate primed pluripotent stem cells. "Embryonic stem cell research largely depends on the use of expensive growth factors, biochemicals and reagents," says Thangaselvam Muthusamy, one of the three lead authors of the study. "Our study describes a process which does not require the addition of any extra factors or chemicals to distinguish pluripotent stem cells. The method also involves the use of already existing equipment - so research labs can easily apply the method with no time or effort involved in learning this new technique. This method is thus both cost-effective and less labor-intensive."
"This work has significant application potential," says Taslimarif Saiyed, Director and COO of the Centre for Cellular and Molecular Platforms, Bangalore. "Novel devices could be developed or new capabilities in existing cell biology devices like flow cyotmerty could be developed based on this principle for applications in stem cell research and eventually for healthcare applications," says Saiyed. So he and Panicker are working towards patenting the method. "We are looking for potential licensee(s) interested in taking the technology to the next step of development," adds Saiyed. "We are already in discussions with a couple of firms interested in licensing this patent."
Apart from its research applications, the work also raises a lot of questions that Panicker and his team look to pursue. "I think the role of lipid bodies, which have been mostly consideredto be storage organelles for lipid molecules, could well be active in signaling and also be 'controlled release devices' within these cells - is an idea worth pursuing," says Panicker. "Also, why they occur when pluripotent stem cells transition from the naïve to the primed state, would be very interesting and important to determine." Their lab is already working towards understanding the signaling mechanisms underlying pluripotency regulated by lipid body-associated proteins.
Panicker says it has been a fun-filled experience. "Lots of people have been working with human pluripotent stem cells since 1998, but we came up with something interesting, useful and unique about these cells primarily from a chance observation and then some follow up experiments along with enjoyable discussions with some of our colleagues" says Mitradas Panicker, faculty at the National Centre for Biological Sciences.
Other scientists on the team agree. "While the observation of fluorescence was purely by chance, determining the chemical nature of the fluorescent molecule i.e. retinyl esters was challenging. The entire research required relentless and continuous teamwork for more than two years but at the end, it is gratifying that the leads generated have immense research potential," says Mukherjee.
The paper was published in Stem Cell Reports and can be accessed here.
Comments
Post new comment